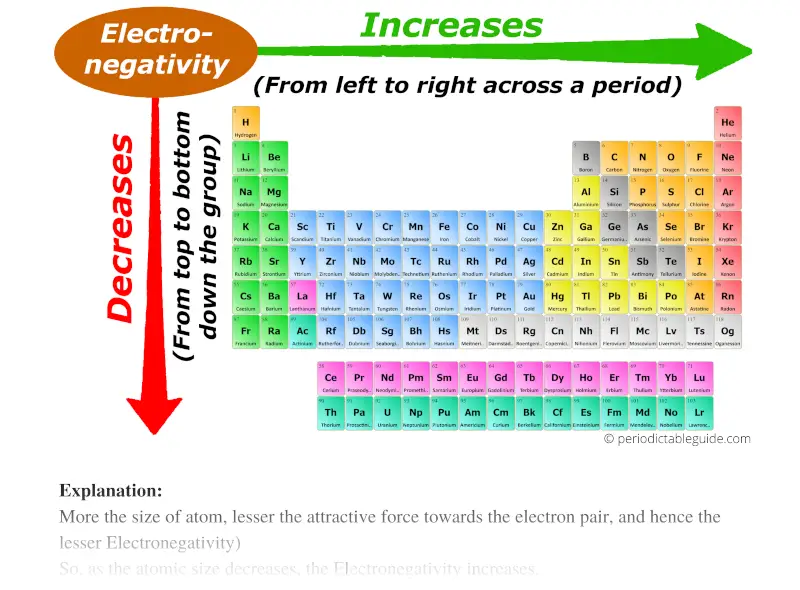
As you go down a group electronegativity decreases. The overall trend for electronegativity in the periodic table is diagonal from the lower left corner to the upper right corner.
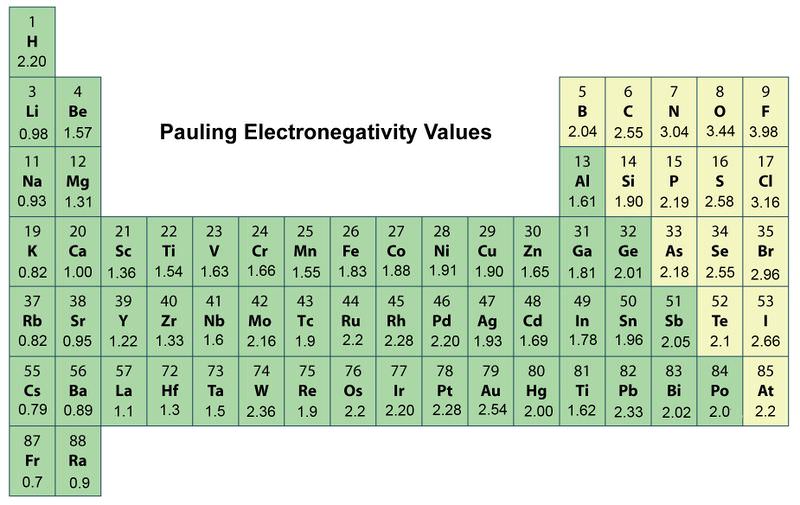
Periodic Trends In Electronegativity Ck 12 Foundation
The trends for electronegativity is that the value increases across the periods rows of the periodic table.

. As we move across a period from left to right the nuclear charge increases and the atomic size decreases therefore the value of electronegativity increases across a period in the modern periodic table. We can see this with the help of a graph showing the trend in electronegativity in period 3 from sodium to chlorine. Why is this part of the periodic trends.
Electronegativity is a measure of the ability of an atom to attract the electrons when the atom is part of a compound. Trends in electronegativity across a period. The electron setup or organization of electrons circling neutral particles demonstrates a common example of periodicity.
Lithium 10 and Fluorine 40 in period 2. For example the electronegativity trend across period 3 in the periodic table is depicted below. Periodic Table Trends.
Electronegativity is the ability of an atom to pull electrons towards it. The electrons involve a progression of electron shells numbered 1 2 etc. Electronegativities generally decrease from top to bottom of a group.
This occurs because of the nuclear charge. In this graph we have not shown argon as it does not react with elements to form bonds. It sees a decreasing trend when you move down a group.
As we move across a period from left to right the nuclear charge increases and the atomic size decreases therefore the value of electronegativity increases across a. The electronegativity also increases up a group column of the periodic table. Electronegativity generally decreases as you go down the periodic table.
Electronegativity increases from left to right of a row as across the row the number of protons in the nucleus increases. As you move from left to right across the periodic table electronegativity increases and as you move down the table electronegativity decreases. As you go across a period the electronegativity increases.
That is as you move across a period the electronegativity increases. When we move from left to right in a period of the modern periodic table electronegativity increases. The atomic number of the elements on the periodic table are organized chronologically starting with Hydrogen with the the atomic number of 1 going from left to right.
What is the periodic trend for electronegativity. It does NOT have any units and is assigned values between 07 and 40. It gradually increases when moving up the periodic tableThis occurs because it is gaining electrons from the covalent bonds it holds with other atoms.
Periodic Trends in the Electronegativities of Elements. Up to 24 cash back Electronegativity has patterns or trends across the periods and the groups. It shows how an atom can swiftly form a chemical bond.
The highest electronegativity value is for fluorine. Which of the following trends is similar to electronegativity follows the same pattern. Electronegativity follows patters similar to that of an atoms ionization energy and electron affinity.
This allows a stronger attraction between outermost electrons and the nucleus. The overall trend for electronegativity in the periodic table is diagonal from the lower left corner to the upper right corner. Electronegativity also decreases as you move down a.
9th - 12th grade. Electronegativity usually rises from left to right. Lithium 10 and Francium 07 in Group I.
When moving from left to right across the periodic table electronegativity increases with the exception being the noble gases. The basic trend for electronegativity across periods is from left to right the electronegativity value tends to increase. Each shell comprises at least one subshell named s p d f and g.
Trends in electronegativity down a group. It doesnt have an electronegativity because it doesnt form bonds. Trends in electronegativity across a period.
In general electronegativity decreases as you move down a group in the periodic table this correlates. Periodic Trends and Patterns 1. Electronegativities generally decrease from top to bottom of a group.
Electronegativity generally increases as you go left to right across the periodic table. Since the electronegativity of some of. Periodic Table Trends.
So electronegativity increases up. The chart shows electronegativities from sodium to chlorine - you have to ignore argon. For example the format of the periodic table is designed so properties can be easily compared.
Up to 24 cash back There are certain trends and patterns in the way elements react and behave. Electronegativity values generally increase from left to right across the periodic table. Todays lab will help identify the electronegativity trend we see on teh Periodic Table.
As mentioned the electronegativity trend refers to the way electronegativity values trend across the periodic table of the elements. However there are exceptions to it at times.
/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)
Easy To Use Chart Of Periodic Table Trends

Electronegativity Definition And Trend

Periodic Trends Made Easy Chemtalk
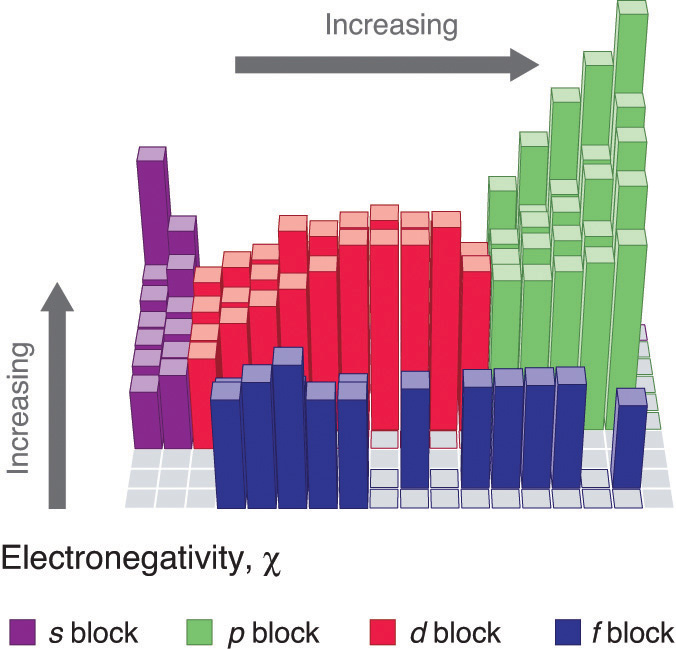
The Periodic Table And Periodic Trends
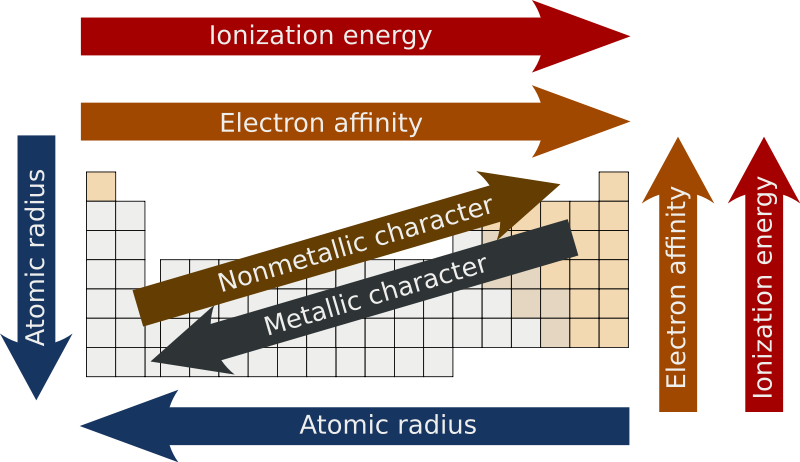

0 comments
Post a Comment